Characteristics of Oxidizers Include Which of the Following
Catalyst oxidizers operate in the same way as thermal oxidizers but with the addition of a catalyst bed. They form coloured ions and compounds.
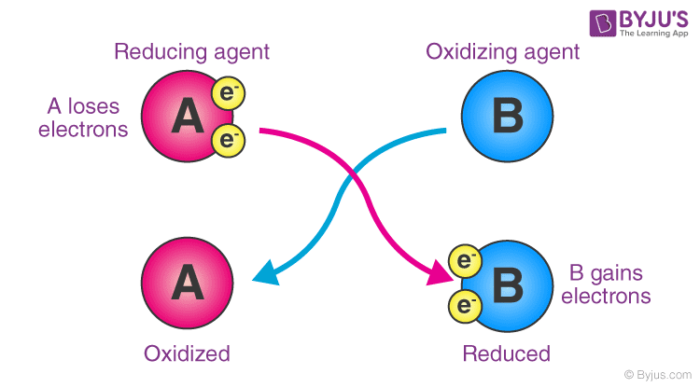
Oxidizing Agent Definition Properties Examples Applications
Select ALL correct statements.

. 1 Have large chargeradius ratio. Heres the chemical properties of oxidizing agents and the examples. The halogens consist of Fluorine Chlorine Bromine Iodine and Astatine.
Examples of chemical properties include flammability toxicity acidity reactivity many types and heat of combustion. These are direct-fired regenerative and recuperative. Is structurally and functionally similar to.
They have a large chargeradius ratio. In the absence of insulin beta-oxidation fatty acid breakdown will increase. There are other chemicals that are oxidizing materials.
Oxidizers are selected to provide the best combination of available oxygen high density low heat of formation low bond energy and maximum gas volume in reaction with binders 17828In addition it should be safe to handle less hygroscopic compatible with. Which of the following is a characteristic that describes nonmetals. The following formula is a reaction that takes place in a specific type of deposition process.
Which response includes all of the following that are displacement reactions and no other reactions. Iron for example combines with oxygen in the presence of water to form rust. Results in higher levels of malate than oxaloacetate.
A characteristic Characteristic determination is accomplished by Testing the waste with the specified test method Applying knowledge of the hazard characteristic in light of the materials or process used Acceptable knowledge includes process knowledge and records of analysis 26211c Characteristic Determination and Testing. 4 Form compounds which are often paramagnetic. If a waste is hazardous because.
Oxidation involves an increase in the oxidation number of an atom. One characteristic of halogens is that its oxidizing quality is weaker from top to bottom. Common examples of oxidizing agents include halogens such as chlorine and fluorine oxygen and hydrogen peroxide H 2 O 2.
Cause an increase in the oxidation state of the substance by making it lose electrons. S2- What it the oxidation state of arsenic in arsenic acid. Characteristic of transition metals.
Wet oxidation of silicon dioxide c. It may or may not also be an oxidation-reduction reaction. The characteristics of oxidizers affect the ballistic and mechanical properties and the processability of a CP.
In which deposition process does this reaction occur. Hydrogen peroxide solutions 8-275. Which of the following species would you expect to have the Largest radius.
There are three main types of thermal oxidizers. A Spheroidal weathering b Red coloration of rocks and soil c Joints in rocks being opened up freezing and thawing d The splitting of rocks by plant roots e Mass wasting. Chromium does not oxidize Figure 2.
Does not burn but is a powerful oxidizer and explosive when mixed with combustible materials. Therefore following an overview of the hazardous waste characteristics regulations this document will present the definition of hazardous waste criteria for identifying hazardous waste and the definitions of each of the four characteristics of hazardous waste. Fluorine is a VIIA group element which is often called as halogens.
Correct option is C Properties of transition elements include. It is ignitable an oxidizer corrosive or reactive when mixed with another waste the resulting mixture is only hazardous if it still displays one of those hazardous waste characteristic s. It may cause eye irritation.
Sodium rubidium potassium cesium a. Oxidizing liquids and solids can be severe fire and explosion hazards. Dry oxidation of silicon dioxide d.
According to this model CO 2 is reduced when it reacts with hydrogen because the oxidation number of the carbon decreases from 4 to 2. 2 Are hard and have high densities. Uses NAD as an electron acceptor.
EXAMPLES OF LIQUID AND SOLID OXIDIZER CLASSIFICATIONS. 5 Arrange the following elements in order of lowest to highest electronegativity. Oxidizers that do not moderately increase1 or cause a slight increase2 in the burning rate of the combustible materials with which they come into contact.
Which of the following indicates oxidation. Increased beta oxidation of fatty acids in the absence of insulin signaling-Insulin signaling causes an increase in fatty acid synthesis. It may produce two elements as products.
7 Form compounds with profound catalytic activity. Thermal oxidizers mainly rely on the oxidation brought about by combustion. Si licon nitride CVD b.
Reduction occurs when the oxidation number of an atom decreases. An oxidizing agent often referred to as an oxidizer or an oxidant is a chemical species that tends to oxidize other substances ie. Characteristics displayed by the original hazardous waste.
5 Show variable oxidation states. They show variable oxidation states which differ by 1 unit. They are hard and have high densities.
6 Form coloured ions and compounds. It is highly reactive and impact or high temperatures can cause violent decomposition or explosion. Liquid air itself has about 30 oxygen which makes it a powerful oxidant.
They form compounds that are often paramagnetic. The following spent non-halogenated solvents. Characteristics of the oxidation of malate to oxaloacetate OAA include all EXCEPT.
For example liquid air has been involved in many explosions because of its oxidizing properties. Which of the following correctly compares fatty acid synthesis and fatty acid oxidation. All inorganic nitrites.
Toluene methyl ethyl ketone carbon disulfide isobutanol pyridine benzene 2-ethoxyethanol and 2-nitropropane. All spent solvent mixturesblends containing before use a total of ten percent or more by volume of one or more of the above non-halogenated solvents or those solvents listed in F001 F002 or F004. Fatty acid uses transport proteins in the mitochondrial membrane while fatty acid oxidation does not.
Spin -on of photoresist Si solid 2H 2 O vapor SiO 2 solid 2H 2 gas 11. It can form shock-sensitive mixtures with finely powdered metals metal oxides strong reducing agents sulfur and phosphorus. They have high melting and boiling points.
Common oxidizing liquids and solids include. 3 Have high melting and boiling points. Increase fatty acid breakdown leads to a buildup of acetyl-CoA which is used to make ketone bodies ketogenesis.
Catalyzed by malate dehydrogenase. Fatty acid synthesis is an endergonic process while fatty acid oxidation is an exergonic process.

Oxidizers 1 What Is An Oxidizer Youtube

Doc Oxidizing Materials Faisal Juli Academia Edu

Oxidizing Potential For Conventional Oxidizing Agents Download Table

Comments
Post a Comment